Lithium-ion batteries will face the risk of excessive self-discharge during long-term storage, especially at lower open-circuit voltages. Due to excessive self-discharge, the voltage of the lithium-ion battery may be too low, causing negative and negative copper foils dissolution and other risks, because the dissolved copper element will be precipitated on the surface of the negative electrode again during the charging process, the generated metal copper dendrites may pierce the separator, causing a short circuit between the positive and negative electrodes, so excessive discharge or low voltage will cause the lithium ion battery completely failed.
At present, we are not particularly clear about the reaction occurring inside the lithium-ion battery during the overdischarge process. In order to understand this reaction process, Johannes Kasnatscheew et al. Of the University of Münster in Germany used a three-electrode system to positive and negative lithium ion batteries during the overdischarge The changes in the polar voltage were studied in detail.
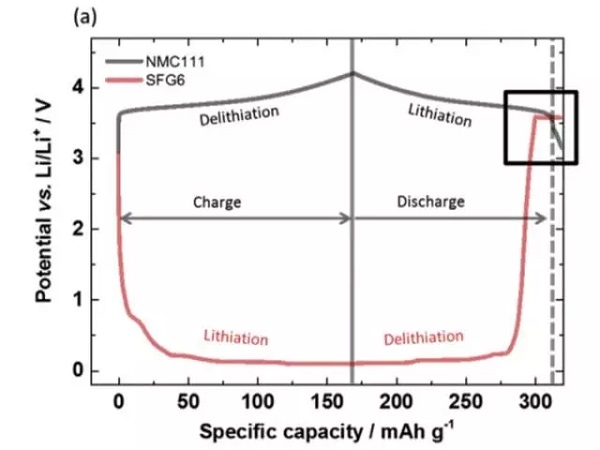
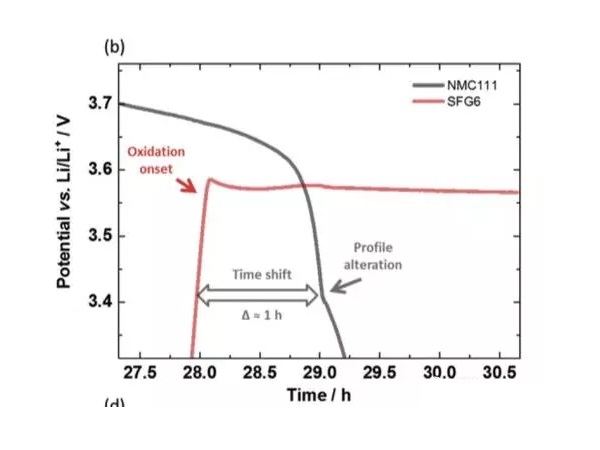
In the experiment, the battery used by Johannes Kasnatscheew et al. Is NMC111 / graphite system, and lithium metal is used as the reference electrode. As can be seen from the following figure a, during the charging process, as Li + is released from the positive electrode, the potential of the positive electrode slowly rises, and the potential of the negative electrode rapidly drops to below 1V. In the process of discharging, Li + is released from the negative electrode Returning to the positive electrode, the potential of the positive electrode decreases gradually. When the negative electrode is completely delithiated, the potential rises rapidly, and a voltage platform appears at about 3.56V. The following figure b is an enlarged view of this area. From the positive and negative voltage curves, you can It can be seen that the change of the positive electrode voltage curve has a delay of about 1h relative to the negative electrode, and then the potential of the positive electrode also begins to rapidly decrease, and the positive electrode potential is lower than that of the negative electrode graphite. This voltage curve change is very consistent with the characteristics of copper foil dissolution. The copper element in copper foil is first oxidized to Cu1 +, which migrates to the surface of the positive electrode and is reduced on the surface of the positive electrode, and is deposited as metallic copper.
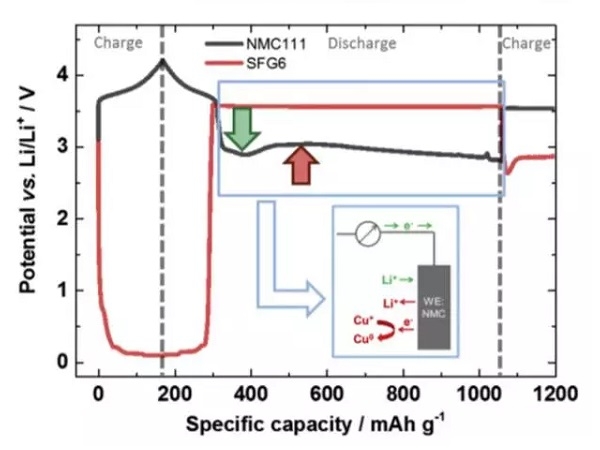
During the entire over-discharge process, the positive and negative electrode potential changes as shown in the figure below. You can see that the negative electrode potential is maintained at about 3.56V, which corresponds to the dissolution of copper foil. The trend of the positive electrode potential is more special. As the copper foil dissolves, the positive electrode potential reaches a minimum, and then there is a slight rebound. Then the positive electrode potential begins to slowly decrease toward the 2.8V cut-off voltage, and lithium insertion causes NMC The potential drop is marked with a green arrow, the voltage drop caused by copper deposition on the NMC surface is marked with a red arrow, and the Li + intercalation reaction and copper deposition occur simultaneously on the positive electrode surface.
As the discharge state changes to the charged state, the potentials of the positive and negative electrodes are reversed, that is, the positive electrode potential is higher than the negative electrode. But we see that the potential of the positive electrode during charging is equivalent to the potential of the negative electrode before the charge and discharge state is reversed, which shows that the reaction at the positive electrode at this time dissolves the copper deposited on its surface again, which also verifies that the negative electrode copper foil is in the process of overdischarge Dissolution occurred and deposition occurred on the surface of the positive electrode.
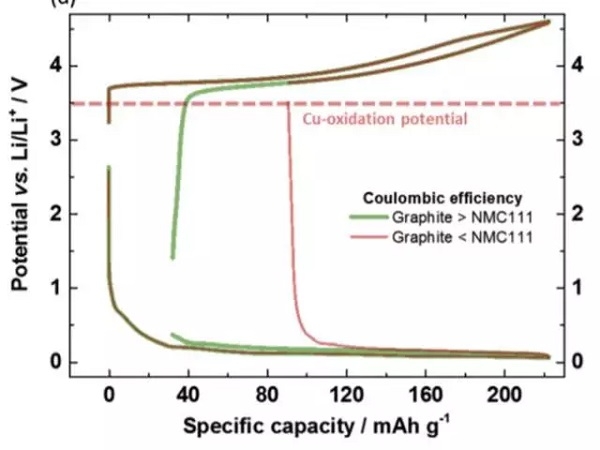
In order to avoid the oxidation and dissolution of the copper foil during the discharge process, it is necessary to control the potential of the negative electrode not higher than 3.56V vs Li + / Li. In the actual process, the potential of the negative electrode is controlled by the battery voltage. The following figure shows the change in the potential during charge and discharge when the first efficiency of the negative electrode is higher than the positive electrode and the first efficiency is lower than the positive electrode. When the first efficiency of the negative electrode is higher than that of the positive electrode, because the positive electrode loses more capacity, although the positive electrode has been completely intercalated with lithium during discharge, the negative electrode still retains part of the lithium, so the potential of the negative electrode is low, and copper foil will not occur. The problem of dissolution. However, when the first efficiency of the negative electrode is low, the positive electrode has not been completely intercalated with lithium during the discharge process. At this time, the lithium of the negative electrode has been consumed, especially when the discharge cut-off voltage is relatively low, it may cause the potential of the negative electrode to be excessive , Leading to the dissolution of copper foil. Therefore, in order to avoid the dissolution of the copper foil, the discharge cut-off voltage of the lithium-ion battery needs to be carefully selected to avoid the potential of the negative electrode being too high.
During the cycle of the battery, as the negative electrode SEI film continues to grow and consume limited Li +, it may exacerbate the negative electrode Li + deficiency, causing its potential to be too high during the discharge process, causing copper to dissolve, so the end of life The cut-off voltage of li-ion batteries is of special concern. Generally speaking, setting a higher discharge cut-off voltage is beneficial to reduce the risk of copper foil dissolution. Therefore, Johannes Kasnatscheew believes that setting the cut-off voltage to 3V can reduce the risk of copper foil dissolution the lowest, improve the cycle life of lithium-ion batteries.



